Considering the need of ISO 17034:2016 Documentation Kit, and how consultants make huge money by selling the papers to organizations just changing the names in those documents instead of real services as expected from consultants, ISOFOLDER is a place where we are trying to make the system effective for documentation for any certification or compliance globally by providing pre-written documentation kit.
About ISO 17034:2016 Documentation Kit
General requirements for the competence of reference material producers. Reference materials (RMs) are used in all stages of the measurement process, including for method validation, calibration and quality control. They are also used in interlaboratory comparisons for method validation and for assessing laboratory proficiency.
No Matter you are planning to get Certified based on international standards e.g. ISO 9001:2015, ISO 14001:2015 etc. or you are asked documentation for compliance e.g. pre-qualifying, legal compliance, tendering, etc. Our ISO 17034:2016 Documentation Kit will facilitate you with less time and cheapest price.
Our ISO 17034:2016 Documentation Kit is written in plain English to make easily customized by you without expertise in standards. We developed a documentation kit while making it user-friendly, easy to learn and time-saving.
We have added sample filled documents, comments of information to be added and other helping tools for you. We are available for lifetime support via email for helping you to make a documentation kit that works for you.
Contents of Documentation Kit
ISO 17034:2016 Documentation Kit is designed in following documents
- ISO 17034 Manual: A sample copy of ISO 17034 Manual which meets the requirements for accreditation of reference material producers. The manual document includes quality policy, organization structure, and macro level system for technical and production requirements.
- Procedures: Total 31 mandatory iso 17034 procedures covering all the specific practice areas of 31 processes that help during iso 17034 certification to make the best system and quick process improvements.
- Exhibits: Total 04 exhibits that covers training requirements and details ideas for process implementation and improvements.
- Work instructions: It includes 23 work instructions for ISO 17034 for establishing a good quality control environment to link with significant aspects issues in the organization.
- Forms and templates: Set of more than 55 standard forms and sample blank templates to maintain records to establish better control.
- ISO 17034 2016 Audit checklist: It covers a set of over 250 audit questions, which can be customized to make your own ISO 17034 audit checklist for internal auditing of your system for quick iso 17034 accreditation.
We have added many documents for reference and use, however, you can add any additional document which is not part of our documentation kit, or can ignore those document which is not applicable to your activities from our documentation kit.
Documentation kit contains A Manual that addresses clause wise details for how management system are designed and implemented in your organization.
A set of procedures, as following, which helps you to manage your operations and address requirements of management system through the implementation of procedures.
- Procedure for establishing the quality of materials as a component of the management system
- Procedure for data evaluation
- Procedure for material handling, storage and transportation
- Procedure for preparation of processing procedures
- Procedure for undertaking characterization
- Procedure for identification, labeling and packaging facilities, packing and delivery
- Procedure for production of reference materials
- Procedure for protection of customer’s confidential information and proprietary rights
- Procedure to avoid involvement in any activities that might diminish confidence in its competence, impartiality, judgment or operational integrity
- Procedure for Document and Data Control
- Procedure for review of request, tender and contracts
- Procedure for subcontracting
- Procedure for procurement of services and supplies
- Procedure for Complaint Handling
- Procedure for Control of non—conforming work and reference materials
- Procedure for Corrective Action
- Procedure for Control of Records
- Procedure for Internal Audit
- Procedure for Management Review
- Procedure for Personnel and Training
- Procedure for production planning and implementation
- Procedure for production control
- Procedure for accommodation and environment
- Procedure for material processing
- Procedure for measurement method
- Procedure for measuring equipments
- Procedure for protecting the integrity of data
- Procedure for metrological traceability
- Procedure for assessment of homogeneity
- Procedure for assessment of stability
- Procedure for assignment of property values and calculation of uncertainty of measurement
A set of work instructions to specify process of micro level activities in organication.
- Work instruction for Chemical Reaction
- Work instruction for Filtration
- Work instruction for Steam Boiler
- Work instruction for Air Compressor
- Work instruction for Water Softening Plant
- Work instruction for Diesel Generator Set
- Work instruction for Spray Drying
- Work instruction for Blending
- Work instruction for Reverse Osmosis Plant
- Work instruction for Pulverizer
- Work instruction for Hot Air Generator
- Work instruction for Ice Plant
- Work instruction for Protection of electronic data
- Work instruction for Preparation of calibration curves
- Work instruction for Handling, Storage, Use of CRM
- Work instruction for Intermediate Check on CRM
- Work instruction for Laboratory Safety
- Work instruction for Disposal method for retained samples
- Work instruction for Spectrophotometer
- Work instruction for Operating Instruction — Weighing balance
- Work instruction for Operating Instruction — Hot Air Oven
- Work instruction for Intermediate checks — Weighing Balance
- Work instruction for Intermediate checks — Oven / Furnace / Dryer
A set of Formats, as the following, which is actually used to implement the system and keep records to provide evidence of the fulfillment of standard requirements.
- Packing Report
- Bill / Invoice
- Customer feedback form
- Complain register
- Preventive Maintenance Schedule
- Non—Conforming Work Report
- Batch manufacturing record
- Indent (purchase requisition)
- Supplier registration form
- Suppliers re—evaluation report
- Sub—contractor audit report
- Stability study report
- Uncertainty of Measurement
- Distilled water generation and test report
- In-house Calibration Report
- Intermediate check report — oven / furnace / dryer
- Critical consumables
- Housekeeping checklist
- Gate Pass
- Change Note
- Master List of Records
- Audit Plan / Schedule
- Clausewise Documentwise Audit Review Report
- Calibration Status of Equipment
- Training Calendar
- Induction Training Report
- Skill Matrix
- Appointment Letter
- Education, training and skill objective
- Delivery challan / deliver memo
- Work order
- Complaint report
- Equipment History Card
- Equipment Wise Preventive Maintenance Checkpoints
- Production Plan
- Purchase Order
- Approved Vendor List
- Inspection report
- Job order
- Environment condition monitoring report
- Re-test Analysis
- Spectrophotometer Calibration Report
- pH Meter Calibration Report
- Intermediate check report — weighing balance
- Intermediate check report — Analyzer
- Normality record sheet
- Sample Test Request Slip
- Master List Cum Distribution List of Documents
- Corrective Action Report
- Quality Objectives
- Internal Audit Non—Conformity Report
- Preventive Action Report
- Internal audit programme
- Training Report
- Job Description and Specification
- Confidentiality Agreement
- Employees Competence Report

 Documentation Kit
Documentation Kit Training Material
Training Material Self-Paced Trainings
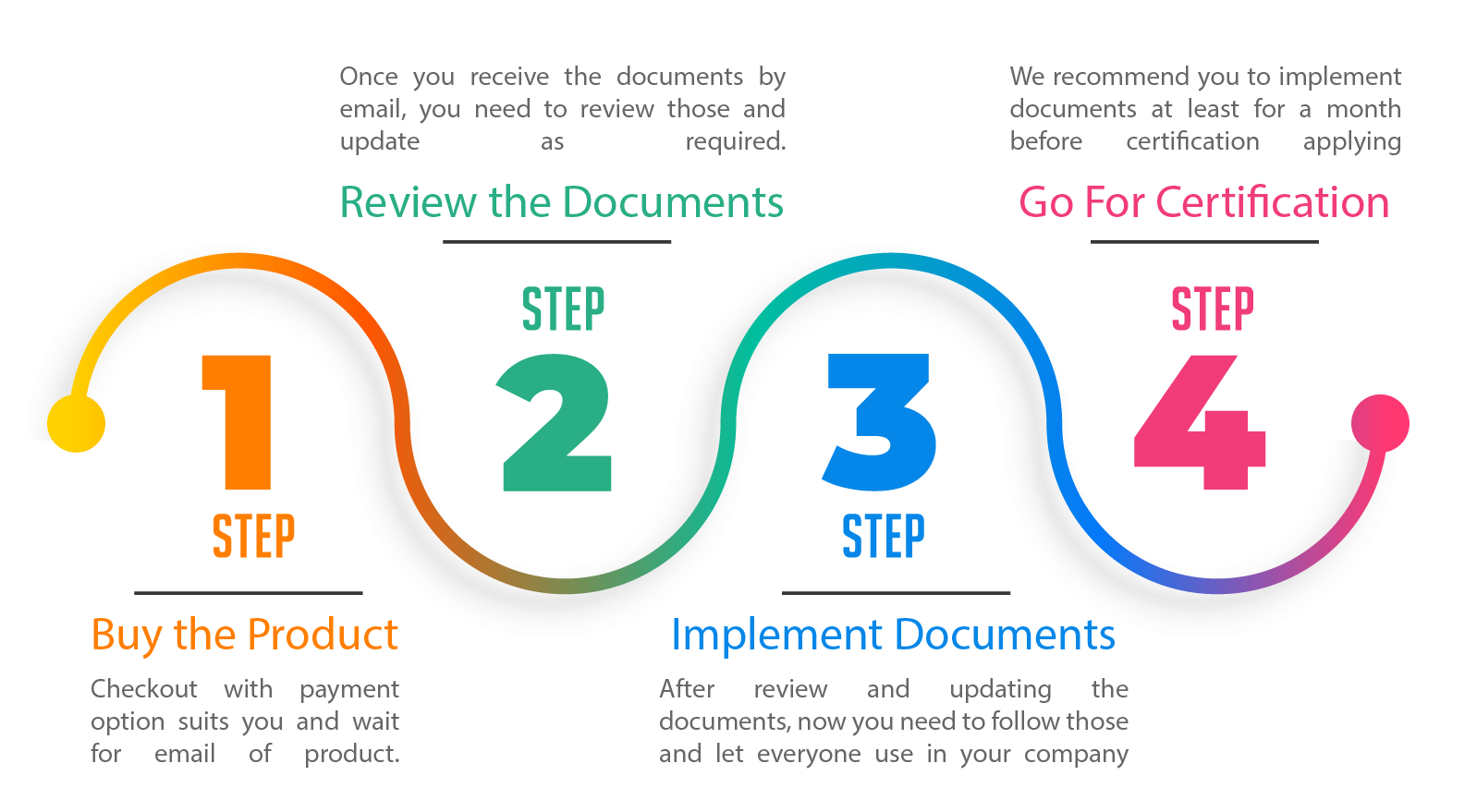
Self-Paced Trainings





Reviews
Clear filtersThere are no reviews yet.