Considering the need of ISO 13485:2016 (MD-QMS) Documentation Kit, and how fake consultants make huge money by selling the papers to organizations just changing the names in those documents instead of real services as expected from consultants, ISOFOLDER is a place where we are trying to make the system effective for documentation for any certification or compliance globally by providing pre-written documentation kit.
ISO 13485 specifies requirements for an organization’s management system manufacturing medical devices.
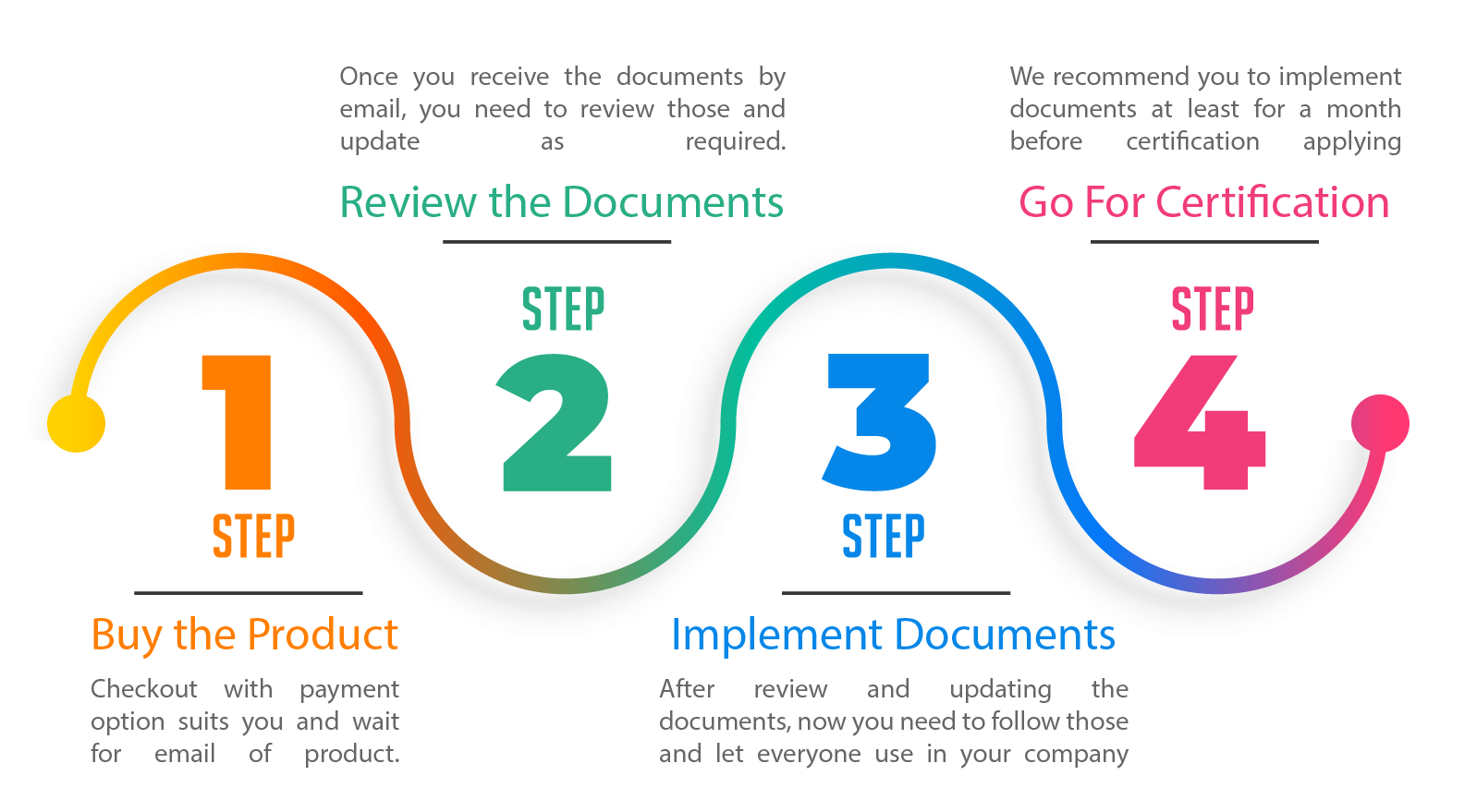
No Matter you are planning to get Certified based on international standards e.g. ISO 9001:2015, ISO 14001:2015 etc. or you are asked documentation for compliance e.g. pre-qualifying, legal compliance, tendering, etc. Our ISO 13485:2016 (QMS) Documentation Kit will facilitate you with less time and cheapest price.
Our ISO 13485:2016 (MD-QMS) Documentation Kit is written in plain English to make easily customized by you without expertise in standards. We developed a documentation kit while making it user-friendly, easy to learn and time-saving.
We have added sample filled documents, comments of information to be added and other helping tools for you. We are available for lifetime support via email for helping you to make a documentation kit that works for you.
Contents of Documentation Kit
Documentation Kit is designed by following four tiers of documents
- TIER 1 – ISO 13485:2016 QUALITY MANAGEMENT SYSTEM MANUAL – This provides the reference to each requirement of the standard. We make the structure of the manual same as the standard so that you can easily understand and follow requirements.
- TIER 2 – ISO 13485 QUALITY MANAGEMENT SYSTEM PROCEDURES (19 nos) – These provide additional information referred in the manual that how you are doing specific activities in your organization
- TIER 3 – FORMATS (51 nos) – These are an actual document that you use to show your compliance with the standard.
We have added many documents for reference and use, however, you can add any additional document which is not part of our documentation kit, or can ignore those document which is not applicable to your activities from our documentation kit.
It is recommended the documentation kit shall be reviewed by senior management, managers, and employees as relevant to them which can help to understand ISO 1385.
The ISO 13485:2016 (MD-QMS) Documentation Kit contains A Quality Manual that address clause wise details for how ISO 1385 systems are designed and implemented in your organization.
A set of procedures, as the following, which helps you to manage your operations and address requirements of ISO 1385 through the implementation of procedures.
- ISO 13485 procedure for Management Review
- ISO 13485 procedure for Control of Document and Record
- ISO 13485 procedure for Internal Audit
- ISO 13485 procedure for Training, Awareness, and Competence
- ISO 13485 procedure for Corrective & Preventive Action
- ISO 13485 procedure for Control of Monitoring and Measuring Equipment
- ISO 13485 procedure for Control of Monitoring of work environment
- ISO 13485 procedure for Monitoring and Measurement of processes
- ISO 13485 procedure for Analyses of Data
- ISO 13485 procedure for Customer Satisfaction Survey
- ISO 13485 procedure for Change Management
- ISO 13485 procedure for Purchasing and Subcontracting
- ISO 13485 procedure for Control of non-conforming products
- ISO 13485 procedure for Objectives and Targets
- ISO 13485 procedure for Legal Compliance
- ISO 13485 procedure for Risk Assessment
- ISO 13485 procedure for Product Identification & Traceability
- ISO 13485 procedure for Product Preservation
- ISO 13485 procedure for Design and Development
A set of Formats, including 51 forms, which is actually used to implement the system and keep records to provide evidence of the fulfillment of standard requirements.
- Purchase Order
- Indent cum Incoming inspection report
- Approved Vendor list cum open purchase order
- Supplier Registration form
- Open Purchase Order
- Daily Stock Statement
- Gate Pass
- Design And Development Plan
- Design Review Minutes Of Meeting
- Design Verification Report
- Design Validation Report
- Breakdown History Card
- Preventive Maintenance Schedule
- Equipment Wise preventive maintenance checkpoints
- Order form/ confirmation
- Customer Complaint report
- Customer Feed Back Form
- Medical Practitioner Feedback Form
- Customer Property Monitoring Register
- Temperature Record
- Validation Of Autoclave By Biological Indicator
- Temperature And Relative Humidity Record (Parentral)
- Temperature And Relative Humidity Record (Washing & Sterilization)
- Temperature And Relative Humidity Record (Filling and Manufacturing)
- Differential Pressure Monitoring Record (Parentral)
- Differential Pressure Monitoring Record (Washing & Sterilization)
- Differential Pressure Monitoring Record (Ointment)
- Temperature & Humidity Monitoring Record — General area
- Microbial Monitoring Of Production Area By Settling Plate Method
- Microbial Monitoring Of Production Area By Settling Plate Method — Ointment preparation
- Microbial Monitoring By Swab “Surface Contact Technique — Parenteral in preparation.
- Microbial Monitoring — Microbial Testing Of Sterile Garments
- Testing Of Personnel By Finger Dab
- Microbial Monitoring By Swab ‘Surface Contact Technique
- Service report
- Repairing card
- Installation commissioning report
- Master List Cum Distribution List Of Documents
- Change Note
- Calibration Status Of Instrument / Equipment
- Master list of records
- Quality Objective Monitoring Report
- Audit Plan I Schedule
- Internal Audit Non—Conformity Report
- Clausewise Documentwise Audit Review Report
- Continual Improvement Plan
- Corrective Action Report
- Preventive Action Report
- Qualitative Process Monitoring Report
- Vendor Rating
- Hazard Analysis Report
- Risk analysis sheet
- Risk indemnification sheet
- Communication report
- Training Calendar
- Training Need Cum Records Sheet
- Induction Training Report
- Job Description and Specification
- Skill Matrix
- Training Report
- Skill Matrix for QC Personnel

 Documentation Kit
Documentation Kit Training Material
Training Material Self-Paced Trainings
Self-Paced Trainings





Reviews
Clear filtersThere are no reviews yet.