Considering the need of HACCP documentation kit, and how fake consultants make huge money by selling the papers to organizations just changing the names in those documents instead of real services as expected from consultants, ISOFolder is a place where we are trying to make the system effective for documentation for any certification or compliance globally by providing pre-written documentation kit.
No Matter you are planning to get certified based on international standards e.g. ISO 9001, ISO 14001, ISO 45001, HACCP, etc. or you are asked documentation for compliance e.g. pre-qualifying, legal compliance, tendering, etc. Our Documentation kit will facilitate you with less time and cheapest price.
Our Documentation Kit is written in plain English to make easily customized by you without expertise in standards. We developed a documentation kit while making it user-friendly, easy to learn and time-saving.
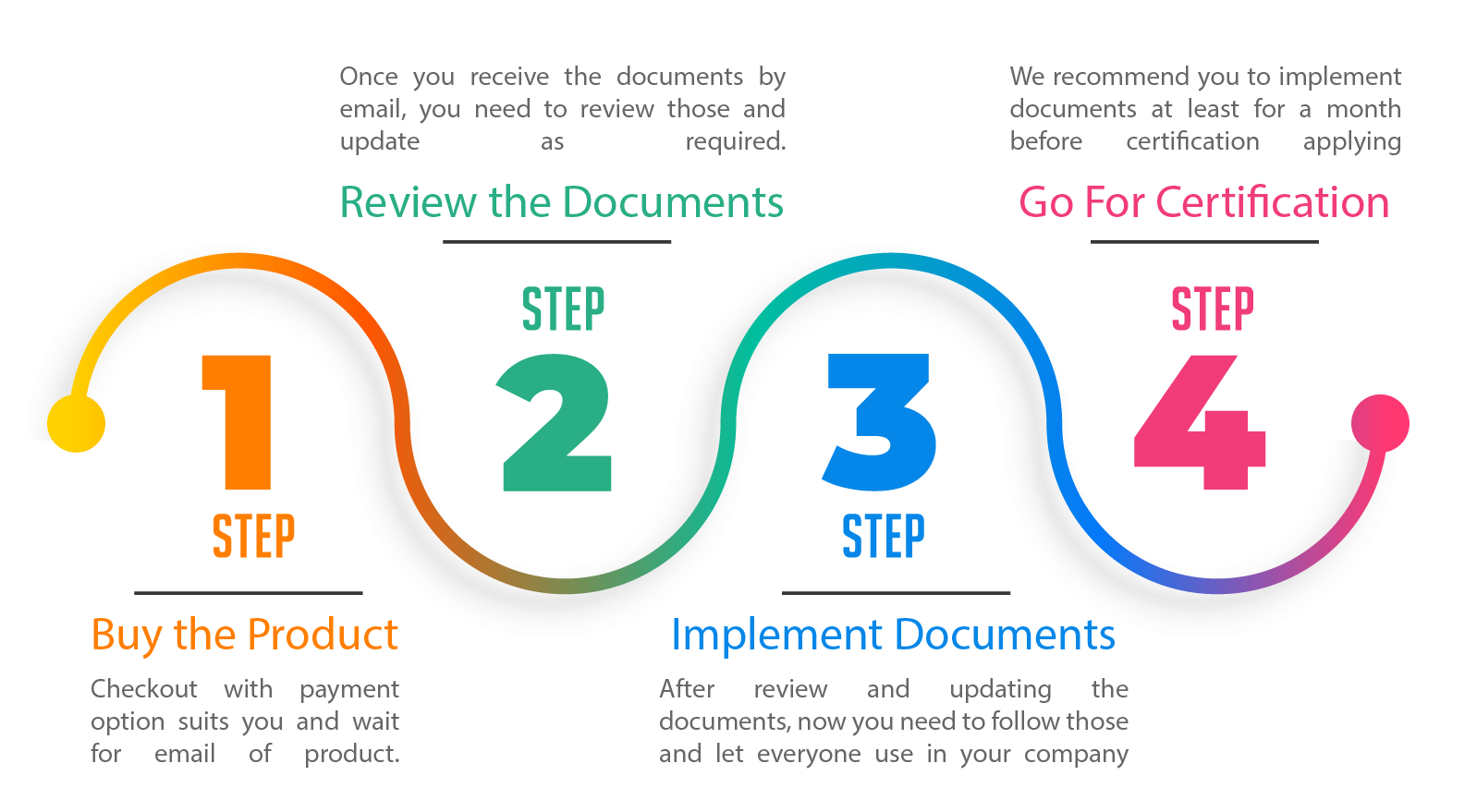
Documentation Kit is designed by following four tiers of documents
- Tier 1 – Manual – This provides the reference to each requirement of the standard. We make the structure of the manual same as the standard, so that you can easily understand and follow requirements.
- Tier 2 – Procedures – This provides additional information referred in the manual that how you are doing specific activities in your organization
- Tier 3 – Formats – These are an actual document that you use to show your compliance with the standard.
We have added many documents for reference and use, however, you can add any additional document which is not part of our documentation kit, or can ignore those document which is not applicable to your activities from our documentation kit.
It is recommended the documentation kit shall be reviewed by senior management, managers, and employees as relevant to them which can help to understand HACCP.
Documentation kit contains a HACCP Manual that address clause wise details for how HACCP systems are designed and implemented in your organization.
17 procedures, which helps you to manage your operations and address requirements of HACCP through the implementation of procedures.
52 Formats , which is actually used to implement the system and keep records to provide evidence of the fulfillment of standard requirements.

 Documentation Kit
Documentation Kit Training Material
Training Material Self-Paced Trainings
Self-Paced Trainings





Reviews
Clear filtersThere are no reviews yet.